
-

Focus Acrylic eclipse 603 Hydrophobic Acrylic
Aspheric Foldable Intraocular Lens
UV & Blue Blocker
About Us -

-


Working closely with technology leading companies as well as leading international surgeons we have been able to assemble a portfolio of unique products and materials to further push the envelope in technological advanced products. When you work with us you can be sure that you will be exposed to new technology and you will see that we are always ready to listen and meet your needs.
When using eyePx products we believe our product users will experience the best possible technology that we can offer with particular attention paid to detail using the highest quality in manufacturing.
In today’s environment of declining prices for surgical reimbursement we want to be looked upon as partners that offer you the best possible price for a high quality product with unique features not offered by others. Quality Policy: Customer Satisfaction Through Continuous Improvement.
ISO 13485:2003
CE all products by LNE/G-MED. France
Free Sale Certification, France
IOLs Models 600, 601 , 602, 603 Focus Acrylic and Focus Acrylic eclipse (photochromic technology by Medennium).
Dispersa, HPMC viscoelastic substance.
Hydrophobic Acrylic Buttons 1.49 Index and 1 .55 Index
Soon to be launched:
Cohesive Viscoelastic. High Molecular Weight HA based viscoelastic.
Adhesa Viscoelastic.
Sutures (nylon, silk, polypropylene, pga).
Cohesive Viscoelastic. High Molecular Weight HA based viscoelastic.
Adhesa Viscoelastic.
Preloaded single piece lens.
Sutures (nylon, silk, polypropylene, pga).


Model 600, designed as a Three Piece Lens of highly biocompatible hydrophobic acrylic material with advanced translucent optics and a UV blocker. The haptics are blue in color manufactured from Polyvinylidene Fluoride (Kynar) with 5 degrees of angulation, modified “C” configuration and a total diameter of 12.5mm designed for implantation in the capsule, or implantation in the sulcus if the capsule has been compromised. The biconvex optic is a full 6.0mm with square edges and it is injectable through a 2.4mm incision using the titanium EL-22M Epsilon Injector in conjunction with the OD502 Ophtec cartridge. For the disposable option, use the Ophtec OD655 cartridge and injector. Available diopters from 0.0 to +30.0
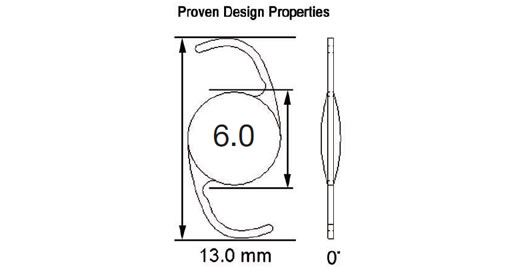

Model 601, designed as a Single Piece Lens of highly biocompatible hydrophobic acrylic material with advanced optics, and UV blocker. The haptic also manufactured from the same acrylic material with 0 degrees angulation and a total diameter of 13.0mm with a full 6.0mm square edge biconvex aspheric optic. The color of the lens is transparent and it is injectable through a 2.2mm incision using the titanium EL-22M Epsilon Injector in conjunction with the OD522 Ophtec cartridge. For the disposable option, use the Ophtec OD665 cartridge and injector or the Comport P by RET Inc. The lens is designed to be positioned in the posterior chamber. Available diopters from 0.0 to +34.0
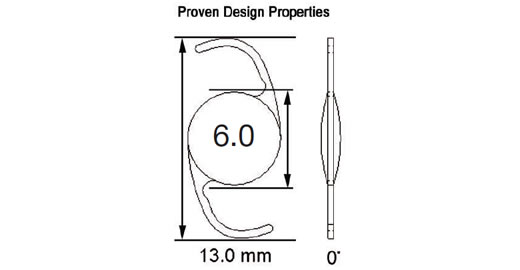


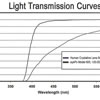
Model 602, designed as a Single Piece Lens of highly biocompatible hydrophobic acrylic material with advanced optics, UV blocker, and Blue Light blocker (50% @ 450 nanometers) designed to protect the retina without compromising mesopic vision. The haptic also manufactured from the same acrylic material with 0 degrees of angulation and a total diameter of 13.0mm with a full 6.0mm square edge symmetric biconvex aspheric optic. The color of the lens is transparent yellow and it is injectable through a 2.8mm to 2.2mm incision using the reusable injectors EL-22M by Epsilon or LI604215 by Medicel with the following cartridges (OD502/OD522 Ophtec or LP604235C Medicel). For the disposable option, use the Ophtec OD665 cartridge/injector. The lens is designed to be positioned in the posterior chamber. Available diopters from -7.0 to +34.0
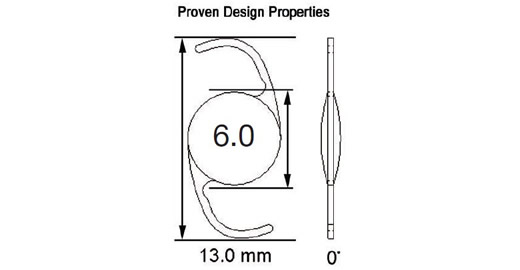
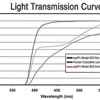

Model 603, designed as a Single Piece Lens of highly biocompatible hydrophobic acrylic material with advanced optics, UV blocker, and Photochromic Blue Light blocker (50% @ 450 nanometers) designed to protect the retina without compromising mesopic vision. The haptic also manufactured from the same acrylic material with 0 degrees of angulation and a total diameter of 13.0mm with a full 6.0 mm square edge biconvex aspheric optic. The color of the lens is transparent yellow when exposed to UV light and it is clear in the absence of UV light. It is injectable through a 2.2mm incision using the titanium EL-22M Epsilon Injector in conjunction with the OD522 Ophtec cartridge. For the disposable option, use the Ophtec OD665 cartridge and injector or the Comport P by RET Inc. The lens is designed to be positioned in the posterior chamber. Available diopters from 0.0 to +34.0

 ELECTRONIC LABELING (DFU)
ELECTRONIC LABELING (DFU)NEW PHOTOCHROMIC FOLDABLE INTRAOCULAR LENS: Preliminary Study of Feasibility and Biocompatibility, Liliana Werner, MD, PhD, Nick Mamalis, MD, Natalya Romaniv, MD, Janathan Haymore, MD, Brian Huagen, MD, Brian Hunter, MD, Scott Stevens, MD, J. Cataract Refract. Surg. 32, 1212-1221 (2006).
First Clinical Experience of Smartvejlow™ (Photochromic IOL by Medennium) in Humans, Guillermo Avalos, Abstract for presentation at ESCRS, London, 2006.
In Vitro and In Vivo Studies for Evaluation of the Matrix Acrylic Aurium Photochromic IOL, Liliana Werner, MD, PhD, Nick Mamalis, MD, Chris Wilcox, Stephen Zhou, PhD, Abstract and PowerPoint presentation at ESCRS, Stockholm, 2007.
First Photochromic Intraocular Lens (Matrix Acrylic™ Aurium)-One Year Experience in Humans: A Comparative Study vs. AcrySof IQ Natural under Progressive Illumination Levels and in Presence or Absence of Sun (UV) Light, David Mendez, MD, Abstract and PowerPoint presentation at ESCRS, Stockholm, 2007.
Matrix Aurium-the First IOL with UV and Blue-Violet Absorption that Shows Photochromic Properties, Preliminary Results, Bordeianu, CD, MD, A PowerPoint presentation.
Lentes Intraoculares Fotocromaticos, Rodolfo Weskamp Irigoyen, MD, Juan Ignacio Irungaray MD, Maria Laura Nuzzolese MD, Cecilia Giorgis, MD, Abstract for XXVII Pan-American Congress of Ophthalmology, Cancun, Mexico, 2007.
Stability of a Novel Photochromic IOL after a Simulated 20 Years in the Eye Using a Nd:Yag Laser Exposure Test, Jacob Brubaker; MD, Ladan Espandar MD, Don Davis Jr. MD, Chris Wilcox, Nick Mamalis, MD, PowerPoint presentation at ASCRS, Chicago, 2008.
Two Years Clinical Experience with the First Photochromic IOL, A Comparative Study, Guillenmo Avalos, MD, PowerPoint presentation at ASCRS, Chicago, 2008.
First Photochromic Hydrophobic Acrylic Intraocular Lens (Aurium™), Stephen Zhou, PhD, Liliana Werner, MD, PhD, David Mendez, MD, abstract for presentation at World Ophthalmology Congress, Hong Kong, 2008.
Ferrari, Francis MD “Un an d’experience avec un implant photochromique”, Pratiques en Ophthalmologie, May 2010, vol4, No 34:92-94 (read full article).
Mingxin Ao MD, Xiaoyong Chen MD, Chen Huang MD, Xuemin Li MD, Zhiquiang Hou MD, Xue Chen MD, Chun Zhang MD, Wei Wang MD, “Color discrimination by patients with different types of light-filtering intraocular lenses”, J Cataract Refract Surg 2010; 36: 389-395.
Heiwei Wang MD, Jun Wang MD, Wenying Fan MD, Wenying Wang MD, “Comparison of photochromic,yellow, and clear intraocular lenses in human eyes under photopic and mesopic lighting conditions”, J Cataract Refract. Surg 2010; 36:2080-2086.
Sebastian Di Cesare, Shawn Maloney, Bruno Fernandes, Claudia Martins, Jean-Claude Marshall, Emilia Antecka, Alexandre N Odashiro, William W Dawson, Miguel N Burnier Jr, “The effect of blue light exposure in an ocular melanoma animal model”, Journal of Experimental and Clinical Cancer Research 2009,28:48(read full article).
Liliana Werner MD PhD, Salwa Abdel-Aziz MD, Carolee Cutler Peck MD, Bryan Monson MD, Ladan Espander, MD, Brian Zaugg, Jack Stringham, Chris Wilcox, Nick Mamalis MD, “Accelerated 20-year sunlight exposure simulation of a photochromic foldable intraocular lens in a rabbit model”, J Cataract Refract Surg 2011; 37: 378-385 c 2011 ASCRS and ESCRS.
Suhas Haldipurkar MD, “Visual and Refractive Outcome in Eyes with Photochromatic IOL”, PowerPoint Presentation, 2011 Laxmi Eye Institute.
A new photochromic IOL has performed very well in initial trials and seems to offer the safety advantages of existing blue-light blocking implants but without compromising mesopic or scotopic vision (read full article).
Until recently, all IOLs on the market had either been transparent and contained a UV-blocking filter or yellow and had the additional ability to filter blue light (read full article).
Your personal information, including your e-mail address and mailing address, will not be sold or rented to a third party. EyePx maintains a strict "no-Spam" policy. When you are on eyePx site and are asked for personal information, you are sharing that information with eyePx and eyePx's authorized distributors only, unless it is specifically stated otherwise. We will use the information you provide for statistical analysis of interest in eyePx. We will use the e-mail address you provide to communicate with you regarding issues of importance. If at any time you wish to be removed from our e-mail list you may opt out by clicking reply and typing the word "cancel" in the subject line of the reply.
If at any time you wish to review, change or update any personal information provided to us, simply send an e-mail to info@eyePx.com. To better protect the security and integrity of your information, we will also take reasonable steps to verify that the communication is from you, before making corrections to your information.
Information that you relate to us will not be posted unless you have granted us permission.
When a visitor requests pages at any eyePx site, our web servers automatically recognize the browser's domain name and IP address, as do most other web servers. This information is collected for statistical and analytical purposes only.
From time to time, eyePx may send a cookie to your computer. This information may be collected to identify your account with the source of advertising that brought you to the site, or to personalize your browsing experience.
If you have questions about our privacy policy, e-mail us at info@eyePx.com. It is our goal to provide a private, risk-free experience for you in interaction with our company.